Medical devices refer to instruments, equipment, appliances, in vitro diagnostic reagents, materials and other similar or related items (required software) that are directly or indirectly used on the human body, and are mainly used for the diagnosis, prevention, monitoring, treatment or treatment of diseases. ease.
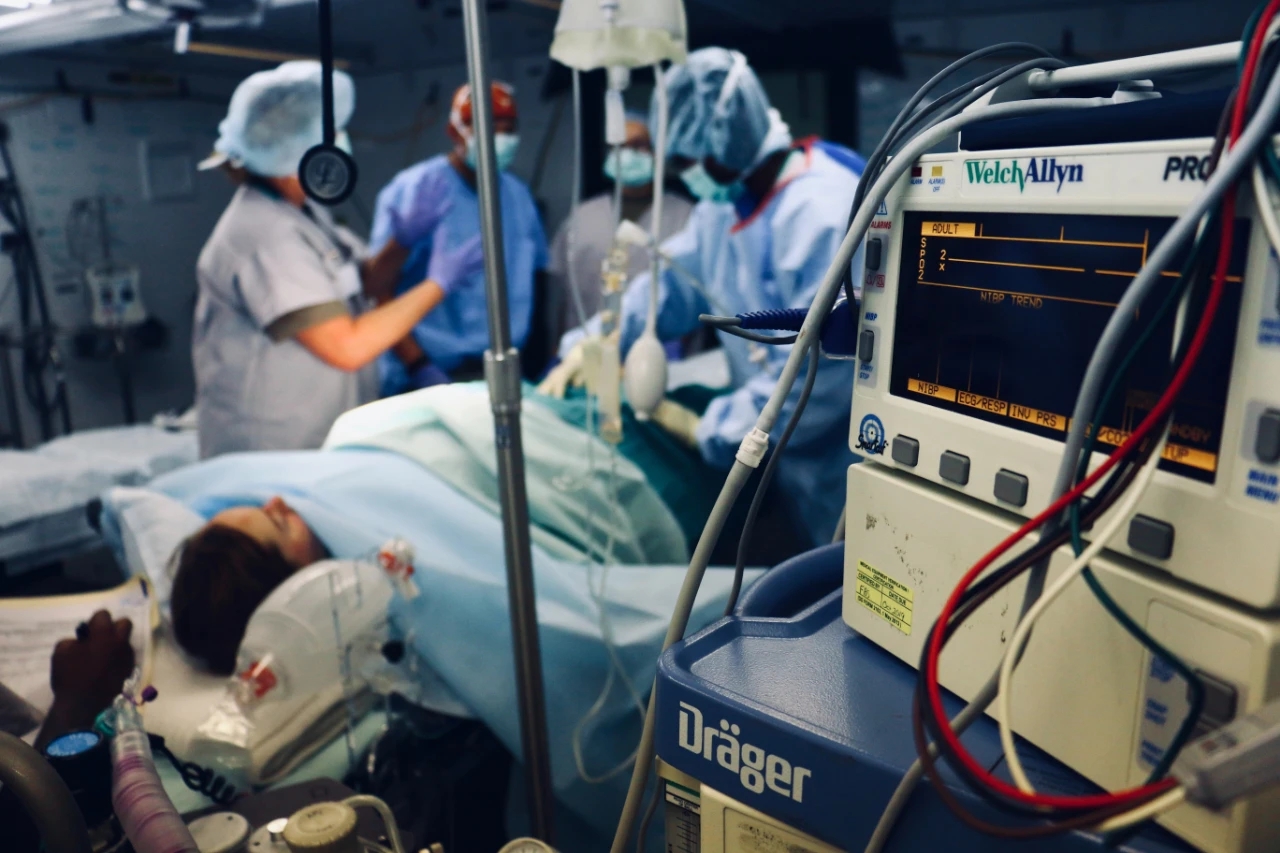
Market size
Medical equipment is a huge market. With the natural growth of the global population, the increase of population aging, the improvement of residents' living standards, and the continuous development of global medical standards, the global medical device market will continue to grow in the long run. Since 2020, affected by the epidemic, medical devices have become one of the most concerned industries.
2016-2020 global medical device industry market scale
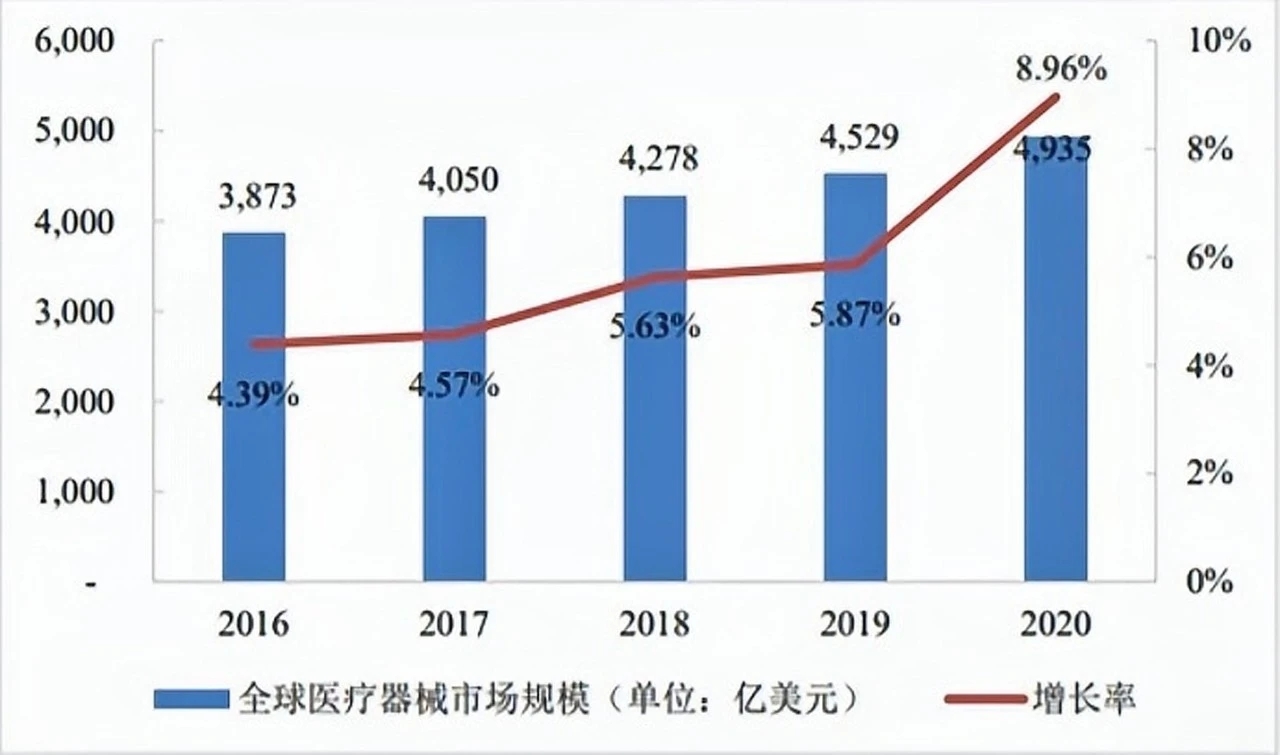
As of the end of 2020, with the exception of China, other countries and regions have not yet reached the end of the epidemic, and their anti-epidemic needs have shifted from short-term to long-term. According to the data published in the "China Medical Device Blue Book (2021 Edition)", the global medical device market size in 2020 will be 493.5 billion US dollars, an increase of 8.96% year-on-year, the market scale continued to expand.
Medical device translation
Medical device translation, including all kinds of medical instruments, medical equipment, medical instruments, medical reagents and calibrators, medical materials and other similar or related medical articles that are directly or indirectly used on the human body.

So what are the precautions for medical device translation?
We can first look at a case: 47 cases of knee replacement surgery failed due to medical device translation errors
In Germany from 2006 to 2007, 47 cases of knee replacement surgery failed due to an incorrect translation of the label of an implantable medical device.
"Professional Translation Magazine" described this case: There are two different types of knee prostheses available, one requires adhesive, and the other does not use adhesive.
On the packaging of this artificial knee joint used in Germany, the source language label says: The femoral prosthesis is "non-modular adhesive", but this information has been incorrectly translated as "non-adhesive" or " No adhesive needed".
For more than a year, the medical professionals and assistants who performed the operation did not discover that they did not correctly implant the knee prosthesis for the patient, and performed the operation by mistake for a total of 47 people.
Knee replacement surgery is a very painful process and usually takes several months to recover. Due to a very basic translation error on the label of the medical device, 47 patients suffered twice as much. In a year's time, they endured two such tortures for no reason.
It can be seen that translation errors of medical devices are not necessarily fatal, but the consequences are likely to be more serious.
Therefore, the translation of medical devices must follow the following two principles:
1. Faithful in content and professional in wording
Medical devices are instruments, equipment and even substances that directly or indirectly act on the human body. Therefore, there are generally clear specifications and strict requirements for medical devices. The contents such as instructions and labels should be consistent with the relevant contents of the registration or filing, and ensure Truth and accuracy, so when providing relevant medical device translation services, it is necessary to maintain the faithfulness of the content and the accuracy and professionalism of the wording.
2. Rigorous structure and rigorous logic
Medical device translation involves the translation of knowledge content in different fields such as medical translation, mechanical translation, electronic translation, plastic translation, etc. It is a high-tech industry of multi-disciplinary knowledge-intensive cross-translation. Its content structure is rigorous and meticulous, and contains a large number of medical professional terms. It is necessary to ensure that the relevant translation content conforms to the medical expression standards, and maintain the rigor of the structure and the rigor of logic.
Translation norms
To sell medical device products internationally, their manufacturers must meet all relevant requirements of the target market, including translation and localization of products and documents. The materials that need to be translated include but are not limited to:
• Marketing materials
• Training materials
• Instructions from medical professionals
• User Guide
• patent application
• Device software and hardware user interface
In addition, the target language and requirements for translation of medical device documents depend on the device type and target market. In the European Union, for example, this may mean providing marketing materials in 24 official and working languages in all member states. In other countries, it is translated into the official language of the target market, and may also need to be translated into minority languages.
The type of equipment is also important. For example, currently, the European Union divides medical devices into four categories based on the degree of risk that medical devices pose to patients, and each category has its own requirements.
The target audience is another factor to consider. In some countries, such as Ireland, the United Kingdom, Cyprus, Luxembourg, Malta, and Poland, English labels for medical equipment are only allowed to be provided when the medical equipment is used by professionals. However, affixing a "Professional Use Only" label on the device does not exempt the manufacturer from liability for injuries caused by improper translation.

Medical equipment is related to the diagnosis, prevention, monitoring, treatment or alleviation of diseases. Professional and high-quality medical equipment translation requires the help of translators with rich medical translation experience and profound professional knowledge to better address the precautions for medical equipment translation. Make a more professional, more reasonable and accurate translation. For more medical device translations, please contact Bianyiyun online customer service.
